General Notes
- FY 2024 funding levels cited in this document are based on the Continuing Resolution in effect at the time of budget preparation (Public Law 118-35) and do not include HIV/AIDS transfers.
- Detail in this document may not sum to the subtotals and totals due to rounding.
Director's Overview

The mission of the National Institute of Neurological Disorders and Stroke (NINDS) is to seek fundamental knowledge about the brain and nervous system and to use that knowledge to reduce the burden of neurological disease for all people. 2025 will mark our 75th anniversary as a leader in neuroscience research, a milestone and opportunity to celebrate our accomplishments and envision progress still to come. Although the sheer complexity of the nervous system and its sensitivity to injury present formidable challenges, decades of basic, translational, and clinical research have led to therapies for previously untreatable conditions and new tools for studying brain development and function across scales from cells to circuits and to behavior. Our achievements give us hope even as the needs remain daunting: neurological disorders are the leading cause of disability and the second leading cause of death worldwide,1 and the impact of conditions such as stroke and neurodegenerative diseases will continue to grow as the population ages. To meet these needs, and guided by the NINDS Strategic Plan, we are investing in innovative and rigorous research, fostering a robust and inclusive research workforce, and working closely with our many partners, including people with lived experience of neurological disorders.
_______________________________________________
1 PubMed: The global burden of neurological disorders: translating evidence into policy
Fueling new approaches to treating neurological disorders
When NINDS was established in 1950, we knew little about stroke, and doctors could do virtually nothing for people who had one. As research identified treatable risk factors such as high blood pressure, stroke mortality began to decline,2 falling 77 percent between 1969 and 2013. Another major advance was the clot-dissolving medicine tissue plasminogen activator (tPA), the first life-saving treatment for acute ischemic stroke. NINDS played a central role in tPA’s success,3 funding early studies providing a rationale for its use and leading pivotal clinical trials supporting Food and Drug Administration (FDA) approval in 1996. NINDS also launched a public campaign to promote urgent treatment for stroke and pioneered protocols for rapid patient assessment and treatment that revolutionized stroke care. This momentum in advancing stroke care has continued, bolstered by research to develop and apply new brain imaging methods and surgical approaches. In 2016, a clinical trial through our stroke trials network (NIH StrokeNet) drove updates to treatment guidelines to expand the use of endovascular thrombectomy (EVT), or surgical clot removal.4
Despite this progress, nearly 800,000 people in the United States have a stroke each year,5 and after decades of decline, stroke mortality rates have plateaued in recent years,6 with concerning increases in some groups and regions. Too few people who experience a stroke meet eligibility requirements for current treatments, and stroke continues to be a leading cause of disability. Looking ahead, a new platform within StrokeNet will simultaneously test several interventions to further optimize EVT, and the Stroke Preclinical Assessment Network (SPAN) is testing candidate drugs that may protect brain tissue and improve outcomes when used with therapies like tPA or EVT to restore blood flow. NINDS also focuses on primary and secondary stroke prevention and on novel approaches to stroke rehabilitation and recovery.
One thousand or more diseases affect the nervous system, including a large number of genetic conditions. Beginning in the 1980s and accelerated by the Human Genome Project, we witnessed an explosion of discoveries pinpointing genetic causes of neurological disorders, enabling faster and more accurate diagnoses and transforming research to understand disease mechanisms and develop targeted therapies. For many neurological diseases, both common and rare, current medications may not achieve their desired outcomes without unintended, off-target side effects. We are now starting to realize the promise of gene-based approaches, such as gene therapies and antisense oligonucleotides (ASO), as more effective and more precise treatments that directly modify disease mechanisms in affected cells and tissues. For example, NINDS-funded research made important contributions to an ASO and a gene therapy for spinal muscular atrophy (SMA), 7, 8 the first FDA-approved therapies for this rare and often fatal condition affecting motor neurons. Gene-based therapies are also approved or in development for other neurological disorders, including muscular dystrophy, and amyotrophic lateral sclerosis (ALS).
To stimulate further advances, NINDS established the Ultra-Rare Gene Therapy Network (URGenT) to support the development of gene-based therapies for ultra-rare diseases, affecting no more than 1 in 50,000 people. Few ultra-rare neurological diseases have effective treatments, and the small number of people affected presents challenges for research, commercial investment, and regulatory approval. Yet, most of these diseases are caused by variations in a single gene, making them excellent candidates for gene-based therapies. In FY 2023, URGenT launched three new projects to develop gene-based therapies for prion disease, Menkes disease, and aspartylglucosaminuria, and a new gene therapy consortium will facilitate clinical trials for ultra-rare diseases as a collaboration within our Network for Excellence in Neuroscience Clinical Trials (NeuroNEXT). URGenT complements NINDS leadership and participation in other genetic therapy initiatives, including the NIH Common Fund’s Somatic Cell Genome Editing and the Bespoke Gene Therapy Consortium through the Accelerating Medicines Partnership (AMP®) managed by the Foundation for the National Institutes of Health (FNIH). These programs have also launched new projects on neurological disorders.
Focusing on gene-based therapies for rare diseases through URGenT and other programs is a first step, and we hope that successes and lessons learned will lead to broader applications for more common neurological conditions as well as diseases that are still unknown. Elusive medical conditions that have not been diagnosed despite extensive clinical evaluation present difficult problems for patients, families, and physicians who may spend years seeking diagnosis and treatment. Leveraging advances in medical and genomic research, the NIH Undiagnosed Diseases Network (UDN) has established exemplary clinical practices, standards, and pipelines for genomics-based diagnoses that can inform patient care. Since its launch by the NIH Common Fund in 2013, the UDN has made over 670 diagnoses including new diseases and syndromes and identified hundreds of disease-linked genes and genomic variants. NINDS has played a major part in the UDN, given that many cases have neurologic involvement, and in FY 2023, the UDN transitioned to NINDS leadership with NIH-wide collaboration to sustain and expand the program to reach a greater number and diversity of participants.
In 1950, neuroscience was a young discipline, and researchers were just beginning to uncover basic principles about how neurons and neural connections work. Mapping of brain circuits and functions was rudimentary, as tools were not available to observe brain structure and activity with sufficient resolution in time or space. Fast-forward to today, and new tools allow research to identify and map individual cell types across the brain,9 monitor thousands of cells in a circuit in real time, precisely manipulate cells’ activity, and non-invasively observe and stimulate the human brain with increasing precision. Made possible by decades of basic neuroscience research, the NIH Brain Research through Advancing Innovative Neurotechnologies® (BRAIN) Initiative—supported by NINDS, the National Institute on Mental Health (NIMH), and eight other NIH partners—has propelled many of these innovations. Researchers are using BRAIN-funded technologies to answer fundamental questions about brain function and disease mechanisms and to develop new diagnostic tools and therapies. Exciting advances include new prosthetics for speech and movement disorders and brain and spinal cord stimulation therapies for chronic pain, Parkinson’s disease, depression, and obsessive compulsive disorder, and motor impairment after stroke. Three large-scale transformative BRAIN projects will build on this progress, including the recently launched BRAIN Initiative Connectivity Across Scales (BRAIN CONNECTS) Network, which will generate brain-wide wiring diagrams.
_______________________________________________
2 PubMed: Temporal Trends in Mortality in the United States, 1969-2013
3 Tissue Plasminogen Activator for Acute Ischemic Stroke (Alteplase, Activase®)
4 Optimizing endovascular therapy for ischemic stroke
5 Centers for Disease Control and Prevention Stroke Facts
6 Office of Disease Prevention and Health Promotion Reduce stroke deaths — Data
7 Nusinersen (Spinraza®) – Spinal Muscular Atrophy (SMA)
8 NIH Director's Blog: Clinical Trials Bring Hope to Kids with Spinal Muscular Atrophy
9 Scientists Unveil Detailed Cell Maps of the Human Brain and the Nonhuman Primate Brain
Partnerships strengthen NINDS research
The challenges and opportunities before us to advance neuroscience research and tackle neurological diseases are too complex for NINDS alone. We rely on the expertise and creativity of the scientific community, and we know that working with our many partners to bring in diverse resources, knowledge, and perspectives will only strengthen and expand the reach of our research. In addition to our roles in the BRAIN Initiative® and the NIH Blueprint for Neuroscience, we bring NINDS leadership and support to NIH-wide activities addressing urgent public health priorities. NINDS co-leads the Researching COVID to Enhance Recovery (RECOVER) Initiative with the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); collaborates with the National Institute on Aging (NIA) to drive research on Alzheimer’s Disease Related Dementias (ADRD); and directs pain management research activities within the Helping to End Addiction Long-term® (HEAL) Initiative, co-led with the National Institute on Drugs and Addiction (NIDA).10 Through the FNIH’s AMP® initiative, we engage in public-private partnerships with industry and non-profit organizations to speed therapy development. AMP® Parkinson’s Disease is working to identify and validate biomarkers for use in diagnosis and prognosis and as tools to aid clinical trials. A new AMP program on ALS will begin soon; this program is one component of a larger partnership established by NIH and the FDA under the Accelerating Access to Critical Therapies for ALS (ACT for ALS) Act, which also includes a new clinical research consortium supported by NINDS to collect natural history data and biospecimens.
We also know that listening to and working with people affected by neurological disorders will lead us to more meaningful progress through research. Consistent with a cross-cutting strategy in the NINDS Strategic Plan, we continue to expand opportunities for community engagement across all stages of research. People with lived experience provide valuable perspectives into AMP® programs and other initiatives; they are informing priorities for a new research roadmap for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS); and they have served on review committees for research using data from expanded access to investigational therapies, a provision of the ACT for ALS. NINDS-funded clinical research studies and networks are also building more community and patient engagement into study development and execution.
_______________________________________________
10 The FY 2025 President’s Budget proposes to rename the National Institute on Drug Abuse to the National Institute on Drugs and Addiction.
Our commitments to diversity, equity, inclusion, and accessibility
NINDS has long recognized that all of neuroscience benefits if we can engage all segments of society in our efforts. The NINDS Strategic Plan includes goals to ensure a vibrant, talented, and diverse neuroscience workforce and to create and sustain a supportive work culture, and it emphasizes overall that diversity, equity, inclusion, and accessibility (DEIA) are essential to the way we work to fund, conduct, and support research. In alignment with these goals, we are working to promote DEIA at NINDS, in research study inclusion, and in other interactions to broaden public participation and community engagement in research. We also take a comprehensive approach to enhancing diversity in the neuroscience workforce across the research career pipeline through programs designed to overcome barriers to participation and inclusion of underrepresented or disadvantaged groups and individuals with disabilities.
Moreover, we are committed to research that will improve health outcomes for every person, and that means doing more to overcome persistent disparities in the prevalence, care, and outcomes, of neurological disorders. In the United States, the risk of stroke is nearly twice as high for Black people as for White people. Despite overall declines in stroke mortality, Black people have the highest rate of death due to stroke, and Hispanic and American Indian/Alaska Native populations have seen the least improvement over time.11 In addition, health disparities exist for other neurological disorders including traumatic brain injury (TBI), pain, epilepsy, and ADRDs, and we do not yet understand the contributing factors. Guided by the NINDS Health Equity Strategic Plan, NINDS is investing in research to understand and eliminate health inequities across neurological disorders, whether associated with race, ethnicity, religion, socioeconomic status, age, mental health, disability, gender, sexual orientation, gender identity, geographic location, or other characteristics historically linked to discrimination, stigmatization, or exclusion. Our new Community-Engaged Health Equity Research in Neuroscience Initiative (HERN) is a multi-pronged approach to understand drivers of health disparities and barriers to equity; develop sustainable interventions; and build capacity to conduct rigorous community-engaged research. NINDS also supports research to address health disparities related to pain and dementia through HEAL and ADRD programs, respectively.
_______________________________________________
11 Source: National Vital Statistics Sample 2008-2018 (latest data available as of Oct 2020)
NINDS Fact Sheet
View the NINDS Fact Sheet(pdf, 584 KB)
Major Changes in the Budget Request
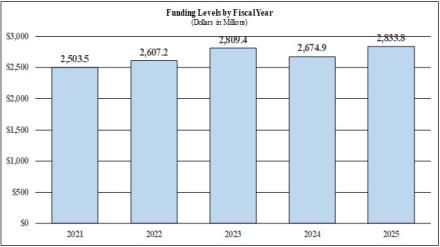
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanism and activity detail, and these highlights will not sum to the total change for the FY 2025 President’s Budget request for the National Institute of Neurological Disorders and Stroke (NINDS), which is $2,833.8 million, an increase of $24.4 million from the FY 2023 Final level. The request includes $45.5 million provided by the 21st Century Cures Act. Within the President’s Budget request level, NINDS will pursue its highest research priorities through strategic investments and careful stewardship of appropriated funds.
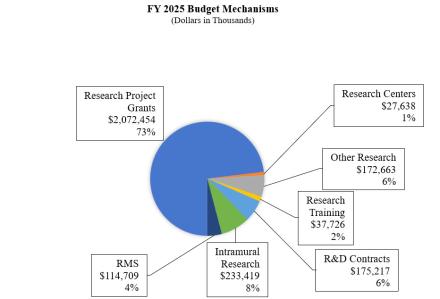
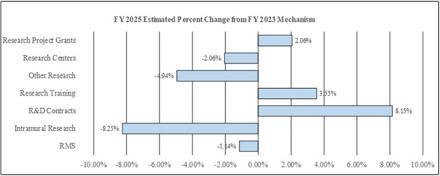
Research Project Grants (RPGs) (+$41.8 million; total $2,072.5 million):
The NINDS budget reflects an increase of $41.8 million in the Research Project Grants portfolio, including Small Business Innovation Research and Small Business Technology Transfer Research (SBIR/STTR) awards, compared to the FY 2023 Final level. RPG awards are expected to increase by 112 grants in FY 2025 compared to the FY 2023 Final level of awards.
Research Centers (-$0.6 million; total $27.6 million):
The NINDS budget reflects a decrease of $0.6 million in the Research Centers portfolio with a reduction of 1 grant in FY 2025 compared to the FY 2023 Final level.
Other Research (-$9.0 million; total $172.7 million):
The NINDS budget reflects a decrease of $9.0 million in the Other Research portfolio, including Research Careers awards. Other Research awards are expected to decrease by 11 awards in FY 2025 compared to the FY 2023 Final level.
Research and Development Contracts (+$13.2 million; total $175.2 million):
The NINDS budget reflects an increase of $13.2 million in Research and Development Contracts including SBIR/STTR awards. NINDS plans to increase R&D contract funding to accommodate new and expanded contracts within translational research programs to develop new drugs, biologic therapies, and devices, including the Blueprint Neurotherapeutics programs.
Intramural Research (-$20.9 million; total $233.4 million):
The NINDS budget reflects a decrease of $20.9 million in Intramural Research. In FY 2023, a catastrophic flood occurred in the Porter Neuroscience Building, resulting in one-time FY 2023 and FY 2024 costs to repair damage and restore lost capital equipment and supplies for 18 NINDS laboratories. This one-time cost is now removed from the Intramural budget for FY 2025.
Research Management and Support (RMS) (-$1.3 million; total $114.7 million):
The NINDS budget reflects a decrease of $1.3 million in RMS to reflect decreased contracting costs and reduced staffing levels for the Helping to End Addiction Long-term (HEAL) Initiative.
Budget Mechanism
Budget Mechanism - Total *, 1
(Dollars in Thousands)
| MECHANISM | FY 2023 Final2 |
FY 2024 Enacted | FY 2025 President's Budget |
FY 2025 +/- FY 2023 |
||||
|---|---|---|---|---|---|---|---|---|
| No. | Amount | No. | Amount | No. | Amount | No. | Amount | |
| Research Projects: | ||||||||
| Noncompeting | 2,327 | $1,265,229 | 2,456 | $1358,426 | 2,620 | $1,481,302 | 293 | $216,073 |
| Administrative Supplements | (272) | $24,427 | (230) | $16,300 | (277) | $18,977 | (5) | -5,450 |
| Competing: | ||||||||
| Renewal | 90 | $60,562 | 80 | $43,568 | 70 | $36,058 | -20 | $24,504 |
| New | 841 | $584,132 | 743 | $415,827 | 678 | $442,147 | -163 | -$141,985 |
| Supplements | 1 | $480 | 0 | 0 | 0 | 0 | -1 | -$480 |
| Subtotal, Competing | 932 | $645,175 | 823 | $459,394 | 748 | $478,205 | -184 | -$166,970 |
| Subtotal, RPGs | 3,259 | $1,934,831 | 3,279 | $1,834,120 | 3,368 | $1,978,483 | 109 | $43,652 |
| SBIR/STTR | 121 | $95,840 | 110 | $85,493 | 124 | $93,971 | 3 | $1,869 |
| Research Project Grants | 3,380 | $2,030,671 | 3,389 | $1,919,613 | 3,492 | $2,072,454 | 112 | $41,783 |
Research Centers: |
||||||||
| Specialized/Comprehensive | 17 | $28,083 | 21 | $34,215 | 16 | $27,638 | -1 | -$444 |
| Clinical Research | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Biotechnology | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Comparative Medicine | 0 | $137 | 0 | 0 | 0 | 0 | 0 | -$137 |
| Research Centers in Minority Institutions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Research Centers | 17 | $28,220 | 21 | $34,215 | 16 | $27,638 | -1 | -$582 |
Other Research: |
||||||||
| Research Careers | 299 | $57,696 | 292 | $62,226 | 311 | $66,615 | 12 | $8,920 |
| Cancer Education | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cooperative Clinical Research | 8 | $1,774 | 9 | $1,102 | 0 | 0 | -8 | -$1,774 |
| Biomedical Research Support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Minority Biomedical Research Support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 258 | $122,161 | 249 | $104,605 | 243 | $106,048 | -15 | -$16,113 |
| Other Research | 565 | $181,631 | 541 | $167,933 | 554 | $172,663 | -11 | -$8,967 |
| Total Research Grants | 3,962 | $2,240,523 | 3,951 | $2,121,761 | 4,062 | $2,272,756 | 100 | $32,234 |
Ruth L. Kirschstein Training Awards: |
FTTPs |
FTTPs |
FTTPs |
FTTPs |
||||
| Individual Awards | 341 | $15,597 | 360 | $16,893 | 370 | $17,167 | 29 | $1,570 |
| Institutional Awards | 312 | $20,837 | 350 | $20,559 | 345 | $20,559 | 33 | -$277 |
| Total Research Training | 653 | $36,434 | 710 | $37,452 | 715 | $37,726 | 62 | $1,292 |
Research & Development Contracts |
139 |
$162,012 |
159 |
$166,943 |
165 |
$175,217 |
26 |
$13,205 |
| SBIR/STTR (non-add) | (9) | ($1,294) | (6) | ($1,333) | (5) | ($1,311) | -(4) | ($17) |
| Intramural Research | 334 | $254,413 | 344 | $235,318 | 348 | $233,419 | 14 | -$20,994 |
| Research Management and Support | 316 | $116,036 | 369 | $113,451 | 381 | $114,709 | 65 | -$1,328 |
| Res. Management & Support (SBIR Admin) (non-add) |
(0) | (837) | (0) | (870) | (0) | (0) | (0) | -($837) |
| Construction | 0 | 0 | 0 | 0 | ||||
| Buildings and Facilities | 0 | 0 | 0 | 0 | ||||
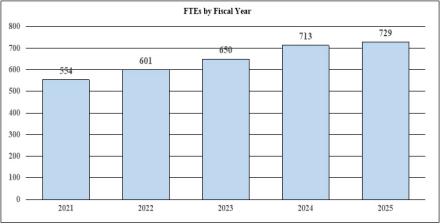
| Total, NINDS | 650 | $2,809,418 | 713 | $2,674,925 | 729 | $2,833,827 | 79 | $24,409 |
* All items in italics and brackets are non-add entries.
1 Of which $225.0 million in FY 2023, $86.0 million in FY 2024, and $45.5 million in FY 2025 is derived by transfer from the NIH Innovation Account under the 21st Century Cures Act.
2 Reflects FY 2023 21st Century Cures Act funding not obligated in FY 2023, and carried over into FY 2024.
Appropriations Language
For carrying out section 301 and title IV of the PHS Act with respect to neurological disorders and stroke, $2,788,327,000.
NIH Innovation Account, Cures Act (Including Transfer of Funds)
For necessary expenses to carry out the purposes described in section 1001(b)(4) of the 21st Century Cures Act, in addition to amounts available for such purposes in the appropriations provided to the NIH in this Act, $127,000,000, to remain available until expended: Provided, That such amounts are appropriated pursuant to section 1001(b)(3) of such Act, are to be derived from amounts transferred under section 1001(b)(2)(A) of such Act, and may be transferred by the Director of the National Institutes of Health to other accounts of the National Institutes of Health solely for the purposes provided in such Act: Provided further, That upon a determination by the Director that funds transferred pursuant to the previous proviso are not necessary for the purposes provided, such amounts may be transferred back to the Account: Provided further, That the transfer authority provided under this heading is in addition to any other transfer authority provided by law.
Summary of Changes
Summary of Changes
(Dollars in Thousands)
| FY 2023 Final |
FY 2025 President's Budget |
Built-in Change from FY 2023 |
||||
|---|---|---|---|---|---|---|
| Changes | FTEs | Budget Authority |
FTEs | Budget Authority |
FTEs | Budget Authority |
| A. Built-in cost changes: 1. Intramural research: |
||||||
| a. FY 2024 effect of FY 2023 pay increase & benefits | $66,853 | $75,117 | $790 | |||
| b. FY 2024 effect of FY 2024pay increase & benefits | $66,853 | $75,117 | $2,601 | |||
| c. FY 2024 paid days adjustment | $66,853 | $75,117 | $257 | |||
| d. Differences attributable to FY 2024 change in FTE | $66,853 | $75,117 | $2,116 | |||
| e. FY 2025 effect of FY 2024 pay & benefits increase | $66,853 | $75,117 | $887 | |||
| f. FY 2025 effect of FY 2025 pay & benefits increase | $66,853 | $75,117 | $1,199 | |||
| g. FY 2025 paid days adjustment | $66,853 | $75,117 | $0 | |||
| h. Differences attributable to FY 2025 change in FTE | $66,853 | $75,117 | $872 | |||
| i. Payment for centrally furnished services | $34,851 | $37,369 | $2,518 | |||
| j. Cost of laboratory supplies, materials, other expenses, and non-recurring costs | $152,701 | $120,932 | $10,376 | |||
| Subtotal, IR built-in cost changes | $21,616 | |||||
| 2. Research Management and Support: | ||||||
| a. FY 2024 effect of FY 2023 pay increase & benefits | $55,754 | $64,642 | $659 | |||
| b. FY 2024 effect of FY 2024pay increase & benefits | $55,754 | $64,642 | $2,169 | |||
| c. FY 2024 paid days adjustment | $55,754 | $64,642 | $215 | |||
| d. Differences attributable to FY 2024 change in FTE | $55,754 | $64,642 | $9,926 | |||
| e. FY 2025 effect of FY 2024 pay & benefits increase | $55,754 | $64,642 | $755 | |||
| f. FY 2025 effect of FY 2025 pay & benefits increase | $55,754 | $64,642 | $1,018 | |||
| g. FY 2025 paid days adjustment | $55,754 | $64,642 | $0 | |||
| h. Differences attributable to FY 2025 change in FTE | $55,754 | $64,642 | $2,331 | |||
| i. Payment for centrally furnished services | $6,047 | $6,484 | $437 | |||
| j. Cost of laboratory supplies, materials, other expenses, and non-recurring costs | $54,231 | $43,583 | $3,082 | |||
| Subtotal, RMS built-in cost changes | $20,592 | |||||
| FY 2023 Final | FY 2025 President's Budget |
Program Change from FY 2023 Final |
||||
|---|---|---|---|---|---|---|
| CHANGES | No. | Amount | No. | Amount | No. | Amount |
| B. Program: 1. Research Project Grants: |
||||||
| a. Noncompeting | 2,327 | $1,289,656 | 2,620 | $1,500,279 | 293 | $210,622 |
| b. Competing; | 932 | $645,175 | 748 | $478,205 | -184 | -$166,970 |
| c. SBIR/STTR | 121 | $95,840 | 124 | $93,971 | 3 | -$1,869 |
| Subtotal, RPGs | 3,380 | $2,030,671 | 3,492 | $2,072,454 | 112 | $41,783 |
| 2. Research Centers | 17 | $28,220 | 16 | $27,638 | -1 | -$582 |
| 3. Other Research | 565 | $181,631 | 554 | $172,663 | -11 | -$8,967 |
| 4. Research Training | 653 | $36,434 | 715 | $37,726 | 62 | $1,292 |
| 5. Research and Development Contracts | 139 | $162,012 | 165 | $175,217 | 26 | $13,205 |
| Subtotal, Extramural | $2,438,969 | $2,485,699 | $46,731 | |||
| 6. Intramural Research | 334 | $254,413 | 348 | $233,419 | 14 | -$42,610 |
| 7. Research Management and Support | 316 | $116,036 | 381 | $114,709 | 65 | -$21,920 |
| 8. Construction | $0 | $0 | $0 | |||
| 9. Buildings and Facilities | $0 | $0 | $0 | |||
| Subtotal, Program changes | -$17,798 | |||||
| Total built-in and program changes | 650 | $2,809,418 | 729 | $2,833,827 | 79 | $24,409 |
Fiscal Year 2025 Budget Graphs
History of Budget Authority and FTE's:
Distribution of Mechanism:
Change by Selected Mechanism:
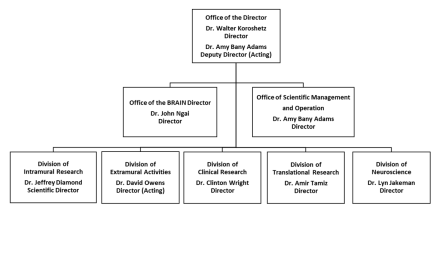
Organization Chart
Budget Authority by Activity
Budget Authority by Activity1
(Dollars in Thousands)
| FY 2023 Final |
FY 2024 CR |
FY 2025 President's Budget |
FY 2025 +/- FY 2023 Final |
|||||
|---|---|---|---|---|---|---|---|---|
| Extramural Research Detail: |
FTEs | Amount | FTEs | Amount | FTEs | Amount | FTEs | Amount |
| Division of Neuroscience | $1,728,133 | $1,627,202 | $1,752,583 | $24,450 | ||||
| Division of Clinical Research | $139,983 | $131,808 | $141,964 | $1,981 | ||||
| Division of Translational Research | $219,373 | $206,560 | $222,477 | $3,104 | ||||
| Division of Extramural Activities | $106,779 | $100,542 | $108,289 | $1,511 | ||||
| Opiod Research2 | $244,700 | $260,044 | $260,386 | $15,686 | ||||
| Subtotal, Extramural | $2,438,969 | $2,326,156 | $2,485,699 | $46,731 | ||||
Intramural Research |
334 | $254,413 | 344 | $235,318 | 348 | $233,419 | 14 | -$20,994 |
| Research Management & Support | 316 | $116,036 | 369 | $113,451 | 381 | $114,709 | 65 | -$1,328 |
| TOTAL | 650 | $2,809,418 | 713 | $2,674,925 | 713 | $2,833,827 | 79 | $24,409 |
1 Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
2 Total for HEAL Initiative including IR and RMS is (in thousands) $280,295 in FY 2023, $280,295 in FY 2024, and $280,295 in FY 2025.
Justification of Budget Request
Justification of Budget Request
National Institute of Neurological Disorders and Stroke
Authorizing Legislation: Section 301 and Title IV of the Public Health Service Act, as amended.
Budget Authority (BA):
| FY 2023 Final |
FY 2024 CR |
FY 2025 President's Budget |
FY 2025 +/- FY 2023 |
|
|---|---|---|---|---|
| BA | $2,809,418,000 | $2,674,925,000 | $2,833,827,000 | $24,409,000 |
| F T E | 650 | 713 | 729 | 79 |
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Overall Budget Policy: The FY 2025 President’s Budget request for NINDS is $2,833.8 million, an increase of $24.4 million or 0.9 percent compared to the FY 2023 Final level. The NINDS request includes 21st Century Cures Act authorized funding of $45.5 million for the BRAIN Initiative, reflecting the Cures Act authorized level for the Brain Research Through Advancing Innovative Neurotechnologies (BRAIN)® Initiative, which is a reduction of $179.5 million from the authorized level in FY 2023. To offset this decrease, the request also includes an increase of $179.5 million in non-Cures Act funding for the BRAIN Initiative to offset the Cures Act reduction and maintain the total FY 2023 Enacted level for the program. Funding for the Helping to End Addiction Long-term (HEAL) Initiative within NINDS is equal to the FY 2023 Final level of $280.3 million. In addition, the request includes an increase of $16.0 million to base funding to support the Undiagnosed Diseases Network (UDN) for a total funding level of $18.0 million within NINDS, in order to complete the transition of the program that started in FY 2023 due to Common Fund policy limiting the duration of its programs.
Program Descriptions
Extramural Research
Investigator-initiated research driven by scientific ingenuity and opportunity is the mainstay of NINDS extramural research and has consistently proven essential to fueling breakthroughs. In addition, specific initiatives focus on priorities that warrant a more targeted approach. NINDS Extramural divisions also support research training, resources, and scientific conferences.
Division of Neuroscience (DON)
As the largest part of the NINDS extramural program, DON supports research on the normal brain, spinal cord, and nerves of the body; mechanisms of neurological injury and disease; and early development of treatments and diagnostics. DON also leads NINDS efforts to promote and leverage data sharing and data science approaches in neuroscience. DON program areas include:
- Basic neuroscience research: Gaps in understanding nervous system development and function hinder progress in treating and preventing neurological disorders. Basic research to fill those gaps does not receive sustained private sector support and is therefore a critical component of the NINDS mission. NINDS works with other NIH Institutes, Centers, and Offices (ICOs) to support all facets of basic neuroscience research, including through the NIH BRAIN Initiative® and the NIH Blueprint for Neuroscience Research. A working group of the National Advisory Neurological Disorders and Stroke Council recently recommended new priorities for fundamental neuroscience research.
- Neurodegeneration: NINDS leads NIH research support for many neurodegenerative diseases, including Alzheimer’s Disease Related Dementias (ADRD), amyotrophic lateral sclerosis (ALS), Parkinson’s disease, movement disorders, and others. This research is making exciting progress toward identifying targets for modifying disease and biomarkers to aid early detection and treatment. Programs include the Morris K. Udall Centers of Excellence in Parkinson's Disease Research and Accelerating Medicines Partnerships for Parkinson’s disease and ALS.
- Stroke and cerebrovascular disease:Among NINDS research on stroke and strategies for neuroprotection and recovery, the Stroke Preclinical Assessment Network (SPAN) rigorously tests potential neuro-protective therapies in rodent models of acute ischemic stroke with replication across multiple labs. SPAN’s first results showed promise for intravenous uric acid, and tests of more candidate therapies are ongoing.12 Vascular contributions to dementia also are a major focus, including studies on white matter disease and on vascular factors involved in a serious side effect of new anti-amyloid therapies for Alzheimer’s disease.
- Rare, genetic, and unknown diseases: Research on the many rare diseases that affect the nervous system meets a critical need and yields insights into other diseases with shared mechanisms. NINDS leads the Undiagnosed Diseases Network (UDN), with plans to expand its reach to include more sites and greater diversity. NINDS initiatives aim to fill gaps in clinical trial readiness for rare neurological and neuromuscular diseases, and NINDS is a partner in the NIH Rare Diseases Clinical Research Network, supporting consortia on lysosomal disorders, mitochondrial diseases, dystonia, neuropathies, and others.
- Other disorders: DON research is informing new treatments and diagnostics for epilepsy, hydrocephalus, spinal cord injury, and traumatic brain injury (TBI), among others. In addition to a leading role in the NIH HEAL Initiative®, NINDS leads pain and headache research and the NIH Pain Consortium (with 21 ICOs). NINDS also collaborates across NIH on areas including developmental conditions like cerebral palsy and autism; muscular dystrophies; autoimmune disorders like multiple sclerosis; nervous system infections including COVID-19; myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS); and brain cancers. Among advances are new insights for treating aggressive brain tumors and growing evidence linking ME/CFS to disruptions in mitochondria and the gut microbiome.
Optimizing Anti-Amyloid Passive Immunotherapies for Dementia As the first disease-modifying drugs approved for Alzheimer’s disease (AD), Aduhelm® and Leqembi® represent significant medical progress. However, a high number (>20%) of participants in clinical trials for these anti-amyloid immuno-therapies developed amyloid-related imaging abnormalities (ARIA), which are adverse events observed on brain imaging that may indicate fluid retention and microbleeds. Most ARIA cases were asymptomatic; but about a quarter showed clinical symptoms, and several patients experienced brain bleeds and swelling.
A leading hypothesis is that ARIA results from anti-amyloid antibodies attaching to amyloid in blood vessel walls, which triggers inflammation. Given safety concerns, patients with known cerebro-vascular pathology have been excluded from clinical trials of anti-amyloid therapies, which could limit their use as more than half of people over 85 with confirmed AD have extensive cerebrovascular damage. Research is needed to understand if these therapies can be modified to be safer, and if they can be effective in people with cerebrovascular and other dementia-related pathologies.
To fill in knowledge gaps for anti-amyloid immunotherapies in AD/ADRD, NINDS is investing in basic, translational, and clinical research to: 1) understand how and why these therapies can damage brain blood vessels, 2) develop strategies that can protect the brain when anti-beta-amyloid immunotherapy is delivered, and 3) support clinical trials of anti-amyloid therapy in unstudied populations, such as patients with vascular pathologies, Lewy bodies, or high levels of beta-amyloid in the brain – patients previously excluded from clinical trials. Clinical trials will be required to include patient and community engagement cores and determine effectiveness in diverse populations.
Patients are already receiving these disease-modifying immunotherapies, yet it is not currently possible to identify or distinguish between patients who may benefit and patients who may be harmed. This research will hopefully provide much needed basic and clinical data and result in strategies to optimize their use.
The Brain Research Through Advancing Innovative Neurotechnologies® (BRAIN) Initiative The NIH BRAIN Initiative® is revolutionizing understanding of the human brain by accelerating the development and application of innovative technologies and ultimately, inspiring new treatments for brain diseases. Spanning 10 NIH Institutes and Centers, the BRAIN Initiative is uniquely positioned for cross-cutting and accelerated discoveries in neuroscience that go beyond the mission of any single ICO. The NIH BRAIN Initiative has invested more than $3 billion in over 1,300 projects that have enabled researchers to understand the brain at unprecedented levels of detail and improve how we treat, prevent, and cure brain disorders. Precise and effective interventions for relieving epilepsy, depression, stroke injury, spinal cord injury, retinal degeneration, pain, opioid use disorder, Parkinson’s disease, and Alzheimer’s disease, for example, are emerging from discoveries about how brain circuits are altered in disease.
The BRAIN Initiative’s focus on accelerating the development and application of innovative technologies is not only laying the foundation for future cures, but also contributing to results in clinical settings today.
- Supported by the BRAIN Initiative and HEAL Initiative, researchers recorded, for the first time, pain-related data from inside the brain of individuals with chronic pain disorders, providing a target for the development of new therapies for chronic pain.1
- The Initiative has fueled the application of deep brain stimulation (DBS) therapy to treat various brain conditions. For example, using DBS therapy, researchers identified a pattern of brain activity, or biomarker, related to the clinical signs of recovery in treatment-resistant depression.2 This is a pivotal step towards using brain data to standardize care, moving promising therapies closer to clinical use.
The BRAIN Initiative launched awards for its third transformative project—the Brain Initiative Connectivity Across Scales (BRAIN CONNECTS) program—which aims to develop a bold, high-resolution wiring diagram of the human brain, helping decipher its neural code. Together with two previously launched transformative projects (the BRAIN Initiative Cell Atlas Network and the Armamentarium for Precision Brain Cell Access), BRAIN CONNECTS promises to transform neuroscience research, illuminating foundational principles governing the circuit basis of behavior and informing new approaches for treating human brain disorders.
__________________________________________________________1 Brain signatures for chronic pain identified in a small group of individuals
2 Researchers discover biomarker for tracking depression recovery
Advancing ALS Research Approximately 30,000 people in the United States have amyotrophic lateral sclerosis (ALS), a rapidly progressive, invariably fatal neurological disease in which motor neurons gradually degenerate, causing progressive weakness and loss of the ability to walk, talk, move, swallow, and eventually breathe. Most people die within three to five years of symptom onset. FDA-approved drugs may slow clinical decline or extend life by several months, and one new therapy targets disease mechanisms in a specific genetic form of ALS. However, no treatments currently stop, reverse, or prevent ALS.
In 2022, the NINDS convened the ALS lived experience and research communities to establish Strategic Research Priorities for ALS. These priorities, which focus on basic, translational, clinical, and quality of life research as well as opportunities for partnerships and collaborations, are guiding programmatic decisions within NINDS and the ALS research community.
With specific appropriations in FY 2022 and 2023 for implementing Accelerating Access to Critical Therapies (ACT) for ALS Act, NIH funded four grants for research utilizing data from expanded access to investigational therapies for ALS and will release new funding opportunities for this program in FY 2024, 2025, and 2026, if funds are available. Additionally, NIH is collaborating with the FDA, industry, nonprofits, and people with lived experience to establish the HHS Public Private Partnership (PPP) for Rare Neurodegenerative Diseases, which is designed to advance research on ALS that will lead to new potential therapies and to build clinical research infrastructure to test those therapies in diverse groups of people with ALS and at risk for ALS. The PPP consists of the Critical Path for Rare Neurodegenerative Diseases (CP-RND) launched in 2022, and the ALS Clinical & Expanded Access Research Consortia and Accelerating Medicines Partnership (AMP®) for ALS launched in 2023. These activities will continue past FY 2025.
Building upon the research priorities identified in the NIH Strategic Priorities for ALS, NIH has commissioned a study from the National Academy of Science, Engineering, and Medicine (NASEM) to recommend actions for the public, private, and non-profit sectors to undertake to make ALS a livable disease within a decade. The study will be completed before October 2024.
Budget Policy: The FY 2025 President’s Budget request for the Division of Neuroscience is $1,752.6 million, an increase of $24.5 million, or 1.4 percent, from the FY 2023 Final level.
__________________________________________________________
12 Researchers develop new method to identify potential stroke therapies
Division of Translational Research (DTR)
DTR leads NINDS extramural development of drugs, devices, and biologic therapies through milestone-driven programs and resources spanning preclinical studies to first-in-human clinical trials. Translational research is prone to failure, and DTR programs help to reduce risks by moving therapies to a point of readiness sufficient for industry interest, or for testing in NINDS-funded clinical trials. Major DTR programs include:
- The Office of Neural Exposome and Toxicology (ONETOX) focuses on non-inherited contributions to neurological diseases, including toxic exposures, social factors, diet, and the microbiome. ONETOX directs the NIH Countermeasures Against Chemical Threats (CounterACT) program, part of the Chemical Countermeasures Research Program at the National Institute of Allergy and Infectious Diseases (NIAID).
- NINDS Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) Programs support research by small businesses on therapies, diagnostics, and research tools, as well as resources to help businesses transition to private funding and commercialization. Recent successes include a new steroid therapy for muscular dystrophy13 and a clinical trial testing a variation of the blood cancer immunotherapy CAR-T to treat myasthenia gravis.14 Many programs in DTR, BRAIN, and HEAL include SBIR/STTR components.
- The NINDS Biomarkers supports development and validation of biomarkers for use in clinical trials to aid therapy development or to help guide patient care decisions. Biomarker projects are in advanced validation stages for multiple sclerosis (MS), traumatic brain injury (TBI), and different forms of Parkinsonism.
- For early stage development of drug or biologic therapies, the Innovation Grants to Nurture Initial Translational Efforts (IGNITE) program funds assay validation, model system development for testing, and proof of concept sufficient for further study. The Epilepsy Therapy Screening Program (ETSP) tests compounds from academic institutions and industry in well-characterized models of epilepsy and has contributed to 11 approved epilepsy drugs.
- For later stage development, the Blueprint Neurotherapeutics Network (BPN), led by NINDS for the NIH Blueprint for Neuroscience Research, supports small molecule drug development, and the Blueprint Neurotherapeutics Network for Biologics (BPN-Biologics) advances biological therapies, including large biological molecules, cell therapies, and gene-targeted therapies. The NINDS Ultra-rare Gene Therapy (URGenT) Network supports precision medicine therapy development for serious ultra-rare neurological diseases affecting no more than 1 in 50,000 people. These diseases represent an important medical need with few available treatments, and they offer promise for advancing genetic therapies for neurological diseases. In FY 2023, URGenT launched three projects to develop new gene-based therapies.
- The NINDS Translational Neural Devices program supports therapeutic and diagnostic device development for nervous system disorders, from preclinical studies through early clinical trials. NINDS also helps to lead NIH-wide support for neural device development through the BRAIN and HEAL initiatives and the Blueprint MedTech program. Since 2015, researchers across these programs filed 39 investigational device exemptions with the FDA to allow clinical studies.
Budget Policy: The FY 2025 President’s Budget request for the Division of Translational Research is $222.5 million, an increase of $3.1 million, or 1.4 percent, from the FY 2023 Final level.
__________________________________________________________
13 NINDS small business funding leads to new therapy for Duchenne muscular dystrophy
14 Potential treatment for rare autoimmune disorder adapted from CAR-T therapy
Division of Clinical Research (DCR)
DCR supports large-scale clinical research and infrastructure, including early and advanced phase clinical trials, epidemiological studies of neurological conditions across the lifespan, comparative effectiveness research, and resources such as the NINDS Common Data Elements, which foster data sharing and data quality. NINDS clinical research networks enable efficient multi-site clinical trials, convene expert communities to promote high-quality research, enable partnerships with industry and people with lived experience, and help train future clinical trial researchers. Major DCR programs include:
- The Network for Excellence in Neuroscience Clinical Trials (NeuroNext) supports Phase II clinical trials to test investigational treatments prior to larger late-stage trials, as well as studies to discover and validate biomarkers. NeuroNEXT will also provide clinical sites and support to facilitate clinical testing of gene-based therapeutics through the NINDS URGenT program. The NIH StrokeNet supports trials on stroke treatment, prevention, and recovery and rehabilitation. StrokeNet research provided key evidence for updated stroke treatment guidelines expanding the use of mechanical clot removal, or endovascular thrombectomy (EVT). StrokeNet has added a new mechanism for platform trials to efficiently and simultaneously test several interventions to further optimize or enhance EVT. Strategies to Innovate EmeRgENcy Care Clinical Trials Network (SIREN), led by NINDS and the National Heart, Lung, and Blood Institute (NHLBI), conducts trials in emergency care for neurologic, cardiac, respiratory, and hematologic conditions.
- Guided by the NINDS Health Equity Strategic Plan, the Office of Global Health and Health Disparities in DCR issued two new funding opportunities for the Community-Engaged Health Equity Research in Neuroscience Initiative (HERN), a multi-pronged approach to advance and build capacity for health equity research across neurological diseases.
Budget Policy: The FY 2025 President’s Budget request for the Division of Clinical Research is $142.0 million, an increase of $2.0 million, or 1.4 percent, from the FY 2023 Final level.
Division of Extramural Activities (DEA)
DEA leads NINDS extramural research training and career development, workforce diversity, and efforts to enhance research rigor, that together enable innovative neuroscience research for generations to come. Major DEA programs include:
- The Office of Programs to Enhance Neuroscience Workforce Diversity (OPEN) promotes the inclusion of underrepresented or historically underserved groups and individuals with disabilities in the neuroscience research workforce. Programs span career stages from K-12 outreach to mentoring networks and initiatives targeting career transitions. For example, OPEN leads the NIH Blueprint and BRAIN Initiative Diversity Specialized Predoctoral to Postdoctoral Advancement in Neuroscience (DSPAN) program to facilitate the transition of graduate students from diverse backgrounds to postdoctoral positions through career development, peer mentoring, and the support of a diverse scientific network. DSPAN has awarded 152 scholars since 2017, and so far, 77 have transitioned to postdoctoral positions and 11 to independent researcher roles.
- The Office of Training and Workforce Development directs NINDS extramural research training and career development, including fellowships, mentored awards, and programs at academic institutions. NINDS initiatives complement NIH-wide programs to address unique training needs across neuroscience research career stages and include national research training programs for neurosurgeons and pediatric neurologists, career development awards for advanced trainees launching independent projects, and workshops focused on strengthening mentorship in neuroscience research. A recent outcomes analysis of the NINDS Research Education Programs for Residents and Fellows in Neurological Disorders and Stroke found that the program increases program graduates’ success in obtaining NIH career development awards (K awards).
- The Office of Research Quality promotes rigor and transparency in neuroscience research and has been instrumental to NIH and research journal policies to improve rigor in experimental design and transparent reporting in publications. A new initiative, Sustainable Transformation of Institutional Research Rigor (STIRR), supports programs to enhance research rigor and transparency practices within academic and research institutions, engaging the research community directly to promote a culture of high-quality neuroscience research.
Budget Policy: The FY 2025 President’s Budget request for the Division of Extramural Activities is $108.3 million, an increase of $1.5 million, or 1.4 percent, from the FY 2023 Final level.
NIH Helping to End Addiction Long-termSM (HEAL) Initiative
The NIH HEAL Initiative® is a trans-agency effort to speed scientific solutions to stem the national opioid crisis. As the NIH lead for pain research, NINDS directs HEAL research to develop effective, non-addictive pain treatments that may reduce reliance on opioids for pain management. NINDS works closely with NIDA on overall HEAL management and on cross-cutting areas, such as addressing opioid use disorder in people with pain; increasing participant diversity and reducing stigma; and leveraging real-time opioid and pain management data. HEAL activities supported through NINDS include other NIH ICOs as partners, and they build on and integrate with NINDS programs for basic, translational, and clinical research.
A suite of milestone-driven translational research programs, including the Pain Therapeutic Development Program, support the discovery, optimization, and development of non-addictive small molecule and biologic therapies for pain. In addition, the Preclinical Screening Platform for Pain tests and characterizes promising therapeutic candidates in animal models that mimic human pain conditions. Parallel programs support development of device-based approaches for pain management, including exciting new technologies to reveal changes in brain activity with chronic pain and use that knowledge to reduce pain through neural stimulation.15NINDS also leads a HEAL program to identify biomarkers that can aid in diagnosing and monitoring pain conditions and improve clinical trials by predicting who might respond to a new therapy.
In clinical research within HEAL, NINDS directs the Early Phase Pain Investigation Network (EPPIC-Net), which provides infrastructure for the rapid design and performance of Phase II clinical trials to test promising pain therapeutics, as well as phenotyping and biomarker studies in people with specific pain conditions. NINDS also plays a lead role in the Pain Management Effectiveness Research Network, which supports clinical trials on the effectiveness of pharmacological and non-pharmacological therapies for pain management. Furthermore, NINDS is a key partner in HEAL programs to reduce health inequities in pain care, with studies to develop, test, and implement interventions to improve outcomes and access to quality pain care in populations that experience health disparities.
For more information, see the section on Chronic Pain and Substance Use Disorder in the Cross-Cutting Initiatives chapter of the NIH Congressional Justification Overview volume.
Budget Policy: The FY 2025 President’s Budget request for NINDS HEAL is $280.3 million, equal to the FY 2023 Final level. NINDS HEAL funding for FY 2025 includes $260.4 million for extramural research, $8.0 million for intramural research, and $11.9 million for research management and support (RMS).
_______________________________________________
15 Pain Relief for Carpal Tunnel Syndrome
Intramural Research Program (IRP)
The NINDS IRP conducts research and research training on the NIH campus. The IRP spans basic, translational, and clinical research in neuroscience, neurology, and neurosurgery and hosts core facilities providing state-of-the-art research technologies. Over 180 labs from NINDS and other Institutes conduct neuroscience research at NIH, including many co-located in the Porter Neuroscience Research Center, creating a rich environment for collaboration that also benefits from the NIH Clinical Center and connections with local hospitals and extramural scientists. NINDS investigators conduct multidisciplinary studies to answer fundamental questions about the nervous system, focusing for example, on the cell biology of neurons, muscle, and glia; neural development and plasticity; and synapses and circuits. Intramural research also includes studies on diseases of the nervous system and early trials for drugs, devices, and gene therapy. Topics of ongoing studies include Gulf War Illness16 (with the Veterans Health Administration), ME/CFS, Long COVID, stroke, neurodegenerative diseases, MS, TBI, epilepsy, brain tumors, movement disorders, and rare genetic disorders. The Roy Blunt Center for Alzheimer’s and Related Dementias (CARD) established by the National Institute on Aging (NIA) and NINDS is a collaborative initiative to initiate, stimulate, accelerate, and support research leading to improved treatment and preventions for these diseases. NINDS investigators also identify causes of puzzling neurological disorders through the Undiagnosed Diseases Program, the intramural component of the UDN.
Among recent advances, NINDS research on ME/CFS and persistent neurological symptoms of Long COVID is pointing to new targets for treating these debilitating and poorly understood conditions. For example, a comprehensive analysis of patients with lasting neurological symptoms after SARS-CoV-2 infection showed broad immune dysregulation in the central nervous system;17 and a detailed examination of post exertional malaise, a key feature of ME/CFS and Long COVID, showed that high levels of a protein called WASF3 disrupt energy production in muscle cells of people with ME/CFS.18 In addition, new findings from studies of vascular injury and repair in the brain identify pathways that might be harnessed for improved outcomes after infections, injury, stroke, neurodegeneration, and autoimmune disease.19
Budget Policy: The FY 2025 President’s Budget request for the Intramural Research Program is $233.4 million, a decrease of $21.0 million, or 8.3 percent, from the FY 2023 Final level.
_______________________________________________
16 VA, NIH launch study of Gulf War Illness
17 NIH study identifies features of Long COVID neurological symptoms
18 Protein may be linked to exercise intolerance in ME/CFS
19 PubMed: Monocyte-derived IL-6 programs microglia to rebuild damaged brain vasculature
Research Management and Support (RMS)
RMS comprises administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research contracts. RMS also includes strategic planning, program evaluation, regulatory compliance, enhanced patient and community engagement activities, internal diversity, equity, inclusion, and accessibility (DEIA) efforts, communication about neurological disorders and NINDS research, and liaison with other agencies, Congress, and the public.
Budget Policy: The FY 2025 President’s Budget request for Research Management and Support is $114.7 million, a decrease of $1.3 million, or 1.1 percent, from the FY 2023 Final level.
Appropriations History
| Fiscal Year | Budget Estimate to Congress | House Allowance | Senate Allowance | Appropriation |
|---|---|---|---|---|
| 2016 | $1,660,375,000 | $1,656,334,000 | $1,694,758,000 | $1,696,139,000 |
| Rescission | $0 | |||
| 20171 | $1,695,180,000 | $1,751,049,000 | $1,803,306,000 | $1,783,654,000 |
| Rescission | $0 | |||
| 20182 | $1,355,998,000 | $1,853,011,000 | $1,904,666,000 | $2,188,149,000 |
| Rescission | $0 | |||
| 20192 | $1,838,556,000 | $2,228,780,000 | $2,275,580,000 | $2,274,413,000 |
| Rescission | $0 | |||
| 20202 | $2,026,031,000 | $2,385,571,000 | $2,490,494,000 | $2,444,687,000 |
| Recission | $0 | |||
| 20212 | $2,245,110,000 | $2,465,110,00 | $2,526,245,000 | $2,513,393,000 |
| Recission | $0 | |||
| 20222 | $2,783,300,000 | $2,799,515,000 | $2,786,096,000 | $2,611,370,000 |
| Recission | $0 | |||
| 20232 | $2,768,043,000 | $2,833,590,000 | $2,765,918,000 | $2,813,925,000 |
| Recission | $0 | |||
| 20242 | $2,825,418,000 | $2,674,925,000 | $2,849,925,000 | $2,674,925,000 |
| Rescission | $0 | |||
| 20252 | $2,833,827,000 |
1 Budget Estimates to Congress includes mandatory financing.
2 Includes funds derived by transfer from the NIH Innovation Account under the 21st Century Cures Act.
Authorizing Legislation
| PHS Act/ Other Citation |
U.S. Code Citation |
2024 Amount Authorized |
FY 2024 CR |
2025 Amount Authorized |
F Y 2025 President's Budget |
|
|---|---|---|---|---|---|---|
| Research and Investigation | Section 301 | 42§241 | Indefinite | $2,674,925,000 | Indefinite | $2,833,827,000 |
| National Institute of Neurological Disorders and Stroke |
Section 401(a) | 42§281 | Indefinite | Indefinite | ||
| Total, Budget Authority | $2,674,925,000 | $2,833,827,000 |
Amounts Available for Obligation
Amounts Available for Obligation 1
(Dollars in Thousands)
| Source of Funding | FY 2023 Final |
FY 2024 Enacted |
FY 2025 President's Budget |
|---|---|---|---|
| Appropriation2 | $2,813,925 | $2,674,925 | $2,833,827 |
| Mandatory Appropriation: (non-add) | |||
| Type 1 Diabetes | ($0) | ($0) | ($0) |
| Other Mandatory financing | ($0) | ($0) | ($0) |
| Subtotal, adjusted appropriation | $2,813,925 | $2,674,925 | $2,833,827 |
| OAR HIV/AIDS Transfers | -$4,507 | $0 | $0 |
| Subtotal, adjusted budget authority | $2,809,418 | $2,674,925 | $2,833,827 |
| Unobligated balance, start of year | $33,120 | $70,249 | $0 |
| Unobligated balance, end of year (carryover)3 | $70,249 | $0 | $0 |
| Subtotal, adjusted budget authority | $2,912,787 | $2,745,174 | $2,833,827 |
| Unobligated balance lapsing | $0 | $0 | $0 |
| Total obligations | $2,912,787 | $2,745,174 | $2,833,827 |
1 Excludes the following amounts (in thousands) for reimbursable activities carried out by this account:
FY 2023 - $36,274 FY 2024 - $38,501 FY 2025 - $40,943
2 Of which $225.0 million in FY 2023, $86.0 million in FY 2024, and $45.5 million in FY 2025 is derived by transfer from the NIH Innovation Account under the 21st Century Cures Act.
3 Reflects 21st Century Cures Act funding not obligated in FY 2023, and carried over into FY 2024.
Budget Authority by Object Class
Budget Authority by Object Class 1
(Dollars in Thousands)
| Total compensable workyears: | FY 2024 CR | FY 2025 President's Budget |
|---|---|---|
| Full-time equivalent | 713 | 729 |
| Full-time equivalent of overtime and holiday hours | 0 | 0 |
| Average ES salary | $201 | $201 |
| Average GM/GS grade | 13.0 | 12.9 |
| Average GM/GS salary | $140 | $144 |
| Average salary, Commissioned Corps (42 U.S.C. 207) | $0 | $0 |
| Average salary of ungraded positions (in whole dollars) | $203 | $209 |
| OBJECT CLASSES | FY 2024 CR | FY 2025 President's Budget |
|---|---|---|
| Personnel Compensation: | ||
| 11.1 Full-time permanent | $51,547 | $54,979 |
| 11.3 Other than full-time permanent | $30,407 | $32,363 |
| 11.5 Other personnel compensation | $3,808 | $4,062 |
| 11.7 Military personnel | $0 | $0 |
| 11.8 Special Personnel Services Payments | $13,118 | $13,485 |
| 11.9 Subtotal Personnel Compensation | $98,880 | $104,889 |
| 12.1 Civilian Personnel benefits | $32,571 | $34,870 |
| 12.2 Military Personnel Benefits | $0 | $0 |
| 13.0 Benefits to Former Personnel | $0 | $0 |
| Subtotal, Pay Costs | $131,452 | $139,760 |
| 21.0 Travel and Transportation of Persons | $3,966 | $4,053 |
| 22.0 Transportation of Things | $267 | $273 |
| 23.1 Rental Payments to GSA | $0 | $0 |
| 23.2 Rental Payments to Others | $94 | $96 |
| 23.3 Communications, Utilities and Miscellaneous Charges | $138 | $141 |
| 24.0 Printing & Reproduction | $8 | $8 |
| 25.1 Consulting Services | $59,129 | $60,965 |
| 25.2 Other Services | $51,697 | $40,939 |
| 25.3 Purchase of goods and services from government accounts | $182,741 | $189,999 |
| 25.4 Operation & Maintenance of Facilities | $154 | $154 |
| 25.5 R&D Contracts | $50,762 | $51,879 |
| 25.6 Medical Care | $732 | $761 |
| 25.7 Operation & Maintenance of Equipment | $8,840 | $9,035 |
| 25.8 Subsistence & Support of Persons | $0 | $0 |
| 25.0 Subtotal Other Contractual Services | $354,056 | $353,732 |
| 26.0 Supplies & Materials | $10,490 | $10,721 |
| 31.0 Equipment | $7,964 | $6,140 |
| 32.0 Land and Structures | $5,049 | $1,575 |
| 33.0 Investments & Loans | $0 | $0 |
| 41.0 Grants, Subsidies & Contributions | $2,161,424 | $2,313,726 |
| 42.0 Insurance Claims & Indemnities | $0 | $0 |
| 43.0 Interest & Dividends | $19 | $19 |
| 44.0 Refunds | $0 | $0 |
| Subtotal Non-Pay Costs | $2,543,473 | $2,694,067 |
| Total Budget Authority by Object Class | $2,674,925 | $2,833,827 |
1 Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
Salaries and Expenses
Salaries and Expenses
(Dollars in Thousands)
| OBJECT CLASSES | FY 2024 CR |
FY 2025 President's Budget |
|---|---|---|
| Personnel Compensation: | ||
| Full-Time Permanent (11.1) | $51,547 | $54,979 |
| Other Than Full-Time Permanent (11.3) | $30,407 | $32,363 |
| Other Personnel Compensation (11.5) | $3,808 | $4,062 |
| Military Personnel (11.7) | $0 | $0 |
| Special Personnel Services Payments (11.8) | $13,118 | $13,485 |
| Subtotal Personnel Compensation (11.9) | $98,880 | $104,889 |
| Civilian Personnel Benefits (12.1) | $32,571 | $34,870 |
| Military Personnel Benefits (12.2) | $0 | v0 |
| Benefits to Former Personnel (13.0) | $0 | $0 |
| Subtotal Pay Costs | $131,452 | $139,760 |
| Travel & Transportation of Persons (21.0) | $3,966 | $4,053 |
| Transportation of Things (22.0) | $267 | $273 |
| Rental Payments to Others (23.2) | $94 | $96 |
| Communications, Utilities and Misc. Charges (23.3) | $138 | $141 |
| Printing and Reproduction (24.0) | $8 | $8 |
| Other Contractual Services: | ||
| Consultant Services (25.1) | $59,062 | $60,898 |
| Other Services (25.2) | $51,697 | $40,939 |
| Purchases from government accounts (25.3) | $107,895 | $109,830 |
| Operation and Maintenance of Facilities (25.4) | $154 | $154 |
| Operation and Maintenance of Equipment (25.7) | $8,840 | $9,035 |
| Subsistence and Support of Persons (25.8) | $0 | $0 |
| Subtotal Other Contractual Services | $227,649 | $220,856 |
| Supplies and Materials (26.0) | $10,490 | $10,721 |
| Subtotal Non-Pay Costs | $242,610 | $236,146 |
| Total Administrative Costs | $374,062 | $375,906 |
Detail of Full-Time Equivalent Employment (FTE's)
Detail of Full-Time Equivalent Employment (FTE's)
| OFFICE/DIVISION | FY 2023 Final |
FY 2024 CR |
FY 2025 President's Budget |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Civilian | Military | Total | Civilian | Military | Total | Civilian | Military | Total | |
| Office of the Director | |||||||||
| Direct: | 77 | - | 77 | 104 | - | 104 | 113 | - | 113 |
| Total: | 77 | - | 77 | 104 | 104 | 113 | - | 113 | |
| Division of Clinical Research | |||||||||
| Direct: | 26 | - | 26 | 28 | - | 28 | 28 | - | 28 |
| Total: | 26 | - | 26 | 28 | - | 28 | 28 | - | 28 |
| Division of Translational Research | |||||||||
| Direct: | 34 | - | 34 | 44 | - | 44 | 44 | - | 44 |
| Total: | 34 | - | 34 | 44 | - | 44 | 44 | - | 44 |
| Division of Intramural Research | |||||||||
| Direct: | 316 | - | 316 | 326 | - | 326 | 330 | - | 330 |
| Total: | 316 | - | 316 | 326 | - | 326 | 330 | - | 330 |
| Division of Extramural Activities | |||||||||
| Direct: | 90 | - | 90 | 96 | - | 96 | 99 | - | 99 |
| Total: | 90 | - | 90 | 96 | - | 96 | 99 | - | 99 |
| Division of Neuroscience | |||||||||
| Direct: | 55 | - | 55 | 63 | - | 63 | 63 | - | 63 |
| Total: | 55 | - | 55 | 63 | - | 63 | 63 | - | 63 |
| Total | 650 | - | 650 | 713 | - | 713 | 729 | - | 729 |
| Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | |||||||||
| FTEs supported by funds from Cooperative Research and Development Agreements | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
FTEs supported by funds from Cooperative Research and Development Agreements.
| FISCAL YEAR | Average GS Grade |
|---|---|
| 2021 | 13.8 |
| 2022 | 12.8 |
| 2023 | 13.0 |
| 2024 | 13.0 |
| 2025 | 12.9 |
Detail of Positions
Detail of Positions 1
(Dollars in Thousands)
| GRADE | FY 2023 Final | FY 2024 CR |
FY 2025 President's Budget |
|---|---|---|---|
| Total, ES Positions | 1 | 1 | 1 |
| Total, ES Salary | $201,135 | $201,135 | $201,135 |
| GM/GS-15 | 84 | 86 | 86 |
| GM/GS-14 | 116 | 122 | 124 |
| GM/GS-13 | 145 | 155 | 160 |
| GS-12 | 71 | 76 | 80 |
| GS-11 | 30 | 32 | 35 |
| GS-10 | 1 | 1 | 1 |
| GS-9 | 15 | 16 | 18 |
| GS-8 | 4 | 4 | 4 |
| GS-7 | 4 | 5 | 5 |
| GS-6 | 2 | 2 | 2 |
| GS-5 | 1 | 1 | 1 |
| GS-4 | 3 | 3 | 3 |
| GS-3 | 1 | 1 | 1 |
| GS-2 | 1 | 1 | 1 |
| GS-1 | 0 | 0 | 0 |
| Subtotal | 477 | 504 | 520 |
| Commissioned Corps (42 U.S.C. 207): | |||
| Assistant Surgeon General | 0 | 0 | 0 |
| Director Grade | 0 | 0 | 0 |
| Senior Grade | 0 | 0 | 0 |
| Full Grade | 0 | 0 | 0 |
| Senior Assistant Grade | 0 | 0 | 0 |
| Assistant Grade | 0 | 0 | 0 |
| Subtotal | 0 | 0 | 0 |
| Ungraded | 207 | 208 | 242 |
| Total permanent positions | 685 | 713 | 499 |
| Total positions, end of year | 685 | 713 | 727 |
| Total full-time equivalent (F T E) employment, end of year | 650 | 713 | 707 |
| Average ES salary | $201,135 | $201,135 | $201,135 |
| Average GM/GS grade | 13.0 | 13.0 | 12.9 |
| Average GM/GS salary | $133,449 | $140,388 | $144,319 |
1 Includes FTEs whose payroll obligations are supported by the NIH Common Fund.
