
NINDS established the Early Phase Pain Investigation Clinical Network (EPPIC-Net) as a part of the NIH HEAL (Helping to End Addiction Long-term) Initiative. EPPIC-Net’s mission is to accelerate and enhance clinical testing of novel, non-addictive pharmacologic and non-pharmacologic therapeutic “assets”, including new and repurposed small molecules, biologics, natural products, and devices, targeted to pain conditions of high unmet need. EPPIC-Net conducts cutting-edge early phase clinical trials across the age and pain condition spectrum of pain therapeutics submitted by industry, academic, and other partners and accepted after rigorous review. EPPIC-Net provides access to a robust clinical trial network with expert infrastructure providing study design, conduct, and analysis at no cost to the applicant. The asset and intellectual property remain with the asset owner.
EPPIC-Net was established in 2019 when a Clinical Coordinating Center, a Data Coordinating Center and 12 Clinical sites were selected through a competitive process (RFA NS-19-023, RFA NS-19-024, RFA NS-19-025). Through discussion with stakeholders, the HEAL Partnership Committee, and the HEAL Multidisciplinary working group, an application process was developed to minimize preparation and submission burden on applicants. Applications are reviewed by a dedicated EPPIC-Net review panel established by the NINDS Science Review Office to assure quality, transparency and fairness in the review process.
EPPIC-Net is NOT currently accepting new applications. Announcements regarding updates to the EPPIC-Net application process and any other relevant HEAL funding opportunities will be posted here as they become available.
EPPIC-Net Goals
EPPIC-Net seeks to enhance the treatment of acute and chronic pain and reduce reliance on opioids.
The goals of EPPIC-Net include:
- Accelerating Phase 2 trials for pain therapeutics
- Incorporating deep phenotyping into the trials
- Providing well-characterized pain patient cohorts
- Ensuring diverse and inclusive representation among researchers and participants
- Continuous learning from experience to engineer adaptive, ever-improving early-phase testing of new pain therapies
To meet these goals, EPPIC-Net:
- Provides stable infrastructure with the capacity to run multiple, high-quality trials concurrently.
- Provides investigators and research staff with expertise across multiple pain conditions
- Manages recruitment to enhance enrollment and minimize conflict between studies focused on similar conditions and similar populations
- Promotes quality assurance
- Supports a bio repository for storage and sharing of samples obtained during the course of EPPIC-Net clinical trials
- Works collaboratively with academia, non-profit, industry, and international partners
The EPPIC-Net Infrastructure
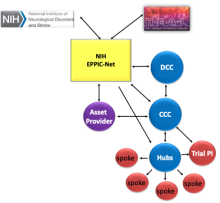
The EPPIC-Net Infrastructure, established through a competitive grant process, includes:
NIH EPPIC-Net – HEAL and NINDS Leadership and EPPIC-Net NIH Program Staff interact with all EPPIC-Net components.
Clinical Coordinating Center (CCC) – The CCC provides overall organizational coordination for all of EPPIC-Net. The CCC takes the lead in protocol development, trial management, site/investigator selection, site/investigator training, reporting and interface with the EPPIC-Net central IRB.
Data Coordinating Center (DCC) – The DCC handles all aspects of data management, statistical support and analysis, quality assurance, and preparation of monitoring reports. The DCC provides the EPPIC-Net biorepository, serving all HEAL programs.
Specialized Clinical Centers (SCCs) – EPPIC-Net has selected 12 Specialized Clinical Centers, each consisting of an academic pain center (hub) and its affiliated clinical locations (spokes). SCC investigators bring specific pain expertise and participate in protocol development and implementation. Hubs and spokes conduct recruit focused pain populations, conduct research procedures, perform local monitoring and submit data to the DCC.
Applicant/Asset Provider: The applicant/asset providers are key collaborators in the EPPIC-Net trial for their asset. They provide the pain therapeutic (drugs/device etc.) for study and retain property rights to asset.
View the EPPIC-Net Coordinating and Specialized Centers contact list
Current Studies
EN20-01: A 24 week study to evaluate the safety and efficacy of CNTX-6970 in subjects with moderate to severe knee osteoarthritis pain
Abstract: The study will employ a randomized, allocation-concealed, multicenter, placebo-controlled, multi-period crossover design (Schmid et al, 2018). This multi-period crossover randomized, controlled trial allows comparability and assessment of efficacy through repeated exposures within each subject to the active treatment and a control (placebo) in randomized sequence. Such multi-period crossover designs are ideal for treatments with rapid onset of action and short half-life such as the asset under study here. We have strived to minimize the complexity of this powerful design by using only 2 blocks with 2 periods each. The modest additional complexity of the proposed multi-period crossover design, compared to a parallel-groups design, is justified by the marked improvement in efficiency. The gains in efficiency afforded by the multi-period crossover design allow a substantial reduction in sample size without sacrificing statistical power. For example, our simulation experiments (with sample sizes ranging from 30 to 50, carryover effects ranging from 0 to 0.2, and an effect size of 0.4) indicated that the parallel design yields statistical power ranging from 0.20-0.25, whereas our proposed 2-block multi-period crossover design yields power ranging from 0.9-1.0.
The trial will compare an active treatment vs. placebo. Each arm of the study will employ a multi-period crossover design with two blocks. Each block will consist of two treatment periods with each period lasting 6 weeks. Treatment assignments (active drug versus placebo) will be randomized for each patient to the two periods within each block. The period length of 6 weeks was chosen based on several considerations: (i) Most efficacious analgesic drugs demonstrate separation from placebo by 6 weeks; (ii) The decision to move CNTX-6970 forward to Phase 3 will require a clinically meaningful separation from placebo by 6 weeks; (iii) In this Phase 2 study, implementing a treatment block longer than 6 weeks would make the overall design more challenging and burdensome by extending the duration of overall testing beyond 6 months; (iv) Recent meta-analyses suggest that anti-inflammatory treatments such as NSAIDs reach peak effects on pain within a 4-week timeframe in patients with knee osteoarthritis, and multiple RCTs have specifically demonstrated efficacy for celecoxib at 2-6 weeks (Osani et al, 2020).
The comparison with celecoxib is used to evaluate "assay sensitivity," i.e., to assess the study protocol's ability to demonstrate superiority of an established efficacious treatment (celecoxib 100mg BID). In this study, the placebo will consist of inactive tablets identical to the active treatment tablets. Treatment assignments (active drug versus placebo) will be randomized for each patient to the two treatment periods within each block
- NCT: NCT05025787
- Status: Completed
- Sites: For more information, visit Enrolling sites
- Principal Investigator: Maurizio Fava, MD Massachusetts General Hospital
EN21-01: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Assess the Safety and Efficacy of 80 mg daily of NRD135S.E1 Versus Placebo in Adult and Elderly Participants with Painful Diabetic Peripheral Neuropathy (SERENDIPITY-1)
Abstract: The purpose of this study is to investigate the safety and efficacy of the current hard gelatin capsule formulation of NRD135S.E1 80 mg once daily in the treatment of PDPN when administered for 13 weeks. This study is an interventional, prospective, parallel-group, multicenter, randomized, double-blind, placebo-controlled, phase 2 study. The study will also evaluate the potential for a rebound effect of the novel oral medication in pain and withdrawal symptoms after IP discontinuation. While it is not the goal of this study to identify the target of the novel oral medication, this trial also provides an opportunity to collect clinical samples that could be used to identify or rule out potential targets.
- NCT: NCT05480228
- Status: Data analysis
- Sites: For more information, visit Enrolling sites
- Principal Investigator: Jessica Robinson-Papp, MD Icahn School of Medicine at Mount Sinai
Painful Diabetic Peripheral Neuropathy (PDPN) Platform Protocol
EPPIC-Net is developing a platform protocol as a framework for Phase 2 assessment of therapeutics for painful diabetic peripheral neuropathy (PDPN). The platform protocol utilizes a modular design with required elements and optional elements customized to asset. Accepted assets are added to the platform protocol as an “Intervention Specific Appendix (ISA).” The platform protocol will be able to test multiple assets individually v. placebo and/or an active control.
Protocol EN21-01 is being conducted under the PDPN platform protocol.
| Module 1 | Screening, Baseline, Daily rating procedures initiation |
|---|---|
| Module 2* | Up-titration and early safety assessment |
| Module 3 | Full study visits |
| Module 4* | Short follow-up visits |
| Module 5* | pK studies |
| Module 6* | Washout |
| Module 7 | Safety follow-up |
The platform protocol will:
- Minimize start-up time
- Promote efficiency through use of common:
- Eligibility criteria, screening procedures
- Data elements
- Equipment
- Training
- Outcome measures (biomarkers, PRO’s, neurologic exam, safety monitoring)
- Controls
- Data harmonization
Training and Mentorship Opportunities
- Notice of Special Interests NOSIs (Close Announcements)
- Open Announcements NOSIs pending.
View the Application Process Webinar
Resources and Tools
Contacts
Please direct EPPIC-Net related questions to: Kevin T. Jones, Ph.D.
Rebecca Hommer, M.D.
Program Director
rebecca.hommer@nih.gov
Jennifer Barnes, Ph.D.
Clinical Project Manager
jennifer.barnes@nih.gov
Kevin T. Jones, Ph.D.
Health Program Specialist
joneskt@nih.gov
eRA Contact
Laura M. Roman, PhD, MBA | Service Desk
Laura.roman@nih.gov
301 451-5966
301 642-4207 (mobile)
EPPIC-Net Coordinating and Specialized Centers Contacts
Funding Opportunities
Open Funding Opportunities/Announcements:
Closed Funding Opportunities/Announcements:
- RFA-NS-24-019 HEAL Initiative: Non-addictive Analgesic Therapeutics Development [Small Molecules and Biologics] to Treat Pain (UG3/UH3 Clinical Trial Optional)
- OTA-22-002(pdf, 181 KB) HEAL Initiative: EPPIC-Net Pain Research Asset Application (OT1)
- NOT-NS-19-075 Notice of Information: HEAL/NINDS Early Phase Pain Investigation Clinical Network (EPPIC-Net): Opening to applications for non-addictive pain therapeutics
- NOT-NS-19-043 Notice of Information: Clinical Trials that Explore Non-Addictive Therapeutics for Pain Conditions under the Early Phase Pain Investigation Clinical Network (EPPIC-Net)
- RFA-NS-19-036 U24 Early Phase Pain Investigation Clinical Network - Specialized Clinical Centers (Reissue of RFA-NS-19-025)
- NOT-NS-19-043 Notice of Information: Clinical Trials that Explore Non-Addictive Therapeutics for Pain Conditions under the Early Phase Pain Investigation Clinical Network
- NOT-NS-19-043 Notice of Information: Clinical Trials that Explore Non-Addictive Therapeutics for Pain Conditions under the Early Phase Pain Investigation Clinical Network
- RFA-NS-19-023 U24 Early Phase Pain Investigation Clinical Network - Clinical Coordinating Center
- RFA-NS-19-024 U24 Early Phase Pain Investigation Clinical Network - Data Coordinating Center
- RFA-NS-19-025 U24 Early Phase Pain Investigation Clinical Network - Specialized Clinical Centers
News & Events
The NIH HEAL Initiative Early Phase Pain Investigation Clinical Network (EPPIC-Net: Updated Application and Review Processes Webinar: March 26, 2021.
Developing Meaningful Endpoints for Pain Clinical Trials
